Renewable Design for Pharmaceutical Intermediate Food Additives D-Ribose CAS 50-69-1
Renewable Design for Pharmaceutical Intermediate Food Additives D-Ribose CAS 50-69-1
Our eternal pursuits are the attitude of “regard the market, regard the custom, regard the science” as well as theory of “quality the basic, have faith in the initial and administration the advanced” for Renewable Design for Pharmaceutical Intermediate Food Additives D-Ribose CAS 50-69-1, We are going to do our greatest to satisfy or exceed customers’ prerequisites with excellent goods, advanced concept, and economical and timely company. We welcome all clients.
Our eternal pursuits are the attitude of “regard the market, regard the custom, regard the science” as well as theory of “quality the basic, have faith in the initial and administration the advanced” for China D-Ribose CAS 50-69-1 and Health Products 50-69-1, What You Want Is What We Pursue.We’ve been sure our goods will bring you first class quality.And now sincerely hope to promote partner friendship with you from all over the world. Let’s joint hands to cooperate with mutual benefits!
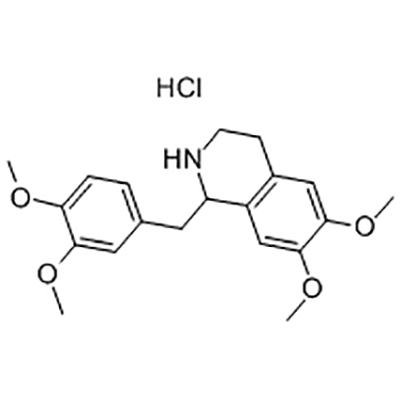
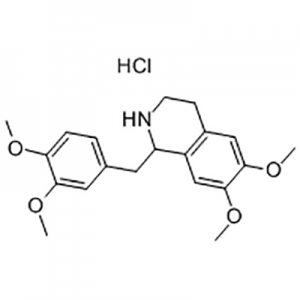
Tetrahydropapaverine hydrochloride is used as the intermediate of Cisatracurium besylate .
Cisatracurium besylate is the benzene sulfonate salt form of atracurium. It is a kind of artificially synthetic non-depolarizing muscle relaxants with its role similar as tubocurarine. It has an onset time of 1 minute and duration time of 15 minutes. The treatment dose does not affect the heart, liver and kidney function. It also has no accumulation property. It also can induce the release of histamine when used at large doses. For muscle relaxation or breathing control required in surgery, compared with current clinical major muscle-relaxing anesthetic drugs, cisatracurium besylate is not metabolized through liver or kidney, and has cardiovascular stability; its effect of muscle relaxation is 3 times as strong as atracurium without any cardiovascular side effects. Cisatracurium besylate is mainly applied to general anesthesia, and can be widely used in intubation, treating liver and kidney dysfunction, used in cardiovascular surgery and elderly and pediatric patients.
Compared with atracurium, this product has no dose-dependent adverse effects of histamine release; however, the disadvantage is that patients with liver and kidney dysfunction should administrate with caution.
Since 1996 for the first time when this drug has entered into market in UK, foreign countries have gradually applied it to replace vecuronium and atracurium as the mainstream of clinical muscle relaxants.
Our eternal pursuits are the attitude of “regard the market, regard the custom, regard the science” as well as theory of “quality the basic, have faith in the initial and administration the advanced” for Renewable Design for Pharmaceutical Intermediate Food Additives D-Ribose CAS 50-69-1, We are going to do our greatest to satisfy or exceed customers’ prerequisites with excellent goods, advanced concept, and economical and timely company. We welcome all clients.
Renewable Design for China D-Ribose CAS 50-69-1 and Health Products 50-69-1, What You Want Is What We Pursue.We’ve been sure our goods will bring you first class quality.And now sincerely hope to promote partner friendship with you from all over the world. Let’s joint hands to cooperate with mutual benefits!