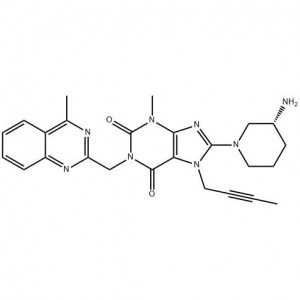
(R)-8-(3-Amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3
(R)-8-(3-Amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3
Linagliptin Linagliptin, developed by Boehringer Ingelheim, was approved by the FDA on May 2, 2011. It is used to combine diet and exercise to improve the control of blood glucose levels in people with type 2 diabetes. Linagliptin improves blood glucose control in patients by inhibiting dipeptidyl peptidase-4 (DPP-4).
Incretin (Incretin) has the effect of promoting the secretion of insulin by pancreatic islet B cells, mainly including glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like polypeptide-1 (GLP-1). DDP-4 is a serine protease located on the surface of cells. It binds to proteins and is widely present in plasma and various tissues in the body (such as kidney, liver, small intestinal villi, endothelial cells, etc.). Linagliptin is a selective DPP-4 inhibitor that inhibits the activity of the enzyme by reversibly binding to DPP-4, delays the degradation of GLP-1, enhances the activity of GLP-1, and stimulates in a glucose-dependent manner Insulin secretes and reduces the level of glucagon in the circulation, thereby regulating the blood glucose level of patients with type 2 diabetes.
Compared with other DPP-4 inhibitors, the main advantages of Linagliptin are: it has excellent renal safety and can effectively reduce glycosylated hemoglobin. Linagliptin is mainly excreted in the feces in the prototype form. After oral administration, the renal excretion is only 5% of the administered amount. Even if it is administered intravenously, only 30.8% is excreted via the kidneys. Therefore, there is no need for patients undergoing treatment. Regularly check liver and kidney function and dose adjustment. All patients can uniformly fix the dose for easy prescription.
Common adverse reactions of Linagliptin are respiratory infection, nasal congestion, muscle pain, headache and sore throat.
Executive standard: enterprise standard
Assay: 98-102%
Exterior: White to yellowish crystalline powder
Package: 25kg/drum

JIN DUN Medical has ISO qualification and meets GMP production standards, employed domestic and foreign drug synthesis experts with rich experience to guide company's R&D.




TECHNOL OGY ADVANTAGES
●High Pressure Catalytic Hydrogenation . High Pressure Hydrogenolysis Reaction . Cryogenic Reaction (<-78%C)
●Aromatic Heterocyclic Synthesis
●Rearrangement Reaction
●Chiral Resolution
●Heck, Suzuki,Negishi,Sonogashira . Gignard Reaction
Equipments
Our Lab has various experimental and testing equipments, such as: NMR (Bruker 400M)、HPLC、chiral-HPLC、LC-MS、LC-MS/MS (API 4000)、IR、UV、GC、GC-MS, Chromatography, Microwave Synthesizer, Parallel Synthesizer, Differential Scanning Calorimeter (DSC), Electron Microscope...
R&D Team
Jindun Medical has a group of professional R&D personnel, and employs many domestic and foreign drug synthesis experts to guide R&D, making our synthesis more accurate and efficient.

We have helped several top domestic pharmaceutical companies, such as Hansoh, Hengrui and HEC Pharm. Here we will show part of them.
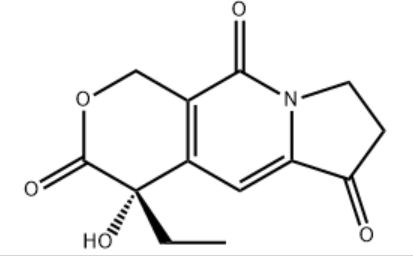
Customzation Case One:
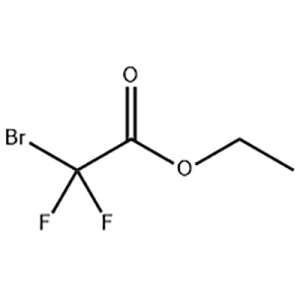
Cas No.: 110351-94-5
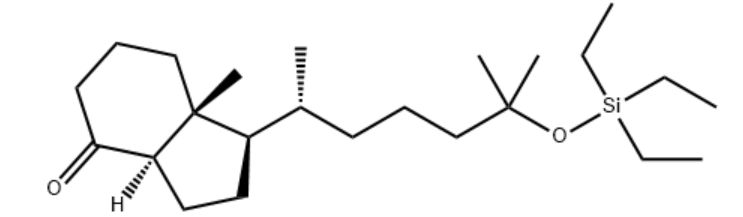
Customzation Case Two:
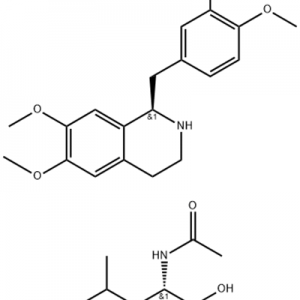
Cas No.: 144848-24-8
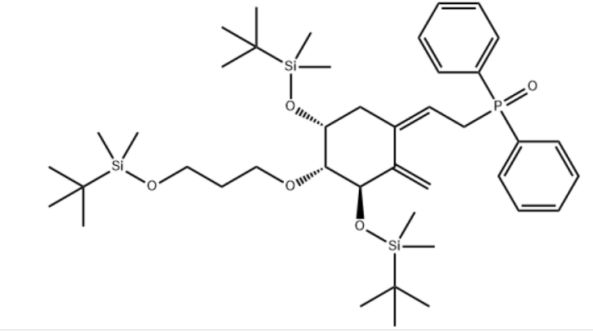
Customzation Case Three:
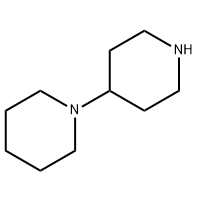
Cas No.: 200636-54-0
1.Customize New Intermediates or APIs. Same as above case sharing, customers have the demands for specific Intermediates or APIs, and they can not find the needed products in the market, then we can help to Customize.
2.Process Optimization for Old Products. Our team will help to Optimize and improve such production whose reaction route is old, production cost is high, and the efficiency is low. We can provide full documentation for technology transfer and process improvement, help customer for a more efficient production.
From drug targets to INDs, JIN DUN Medical provides you with one-stop personalized R&D solutions.
JIN DUN Medical insists on creating a team with dreams, making dignified products, meticulous, rigorous, and going all out to be a trusted partner and friend of customers!



![pentamethylene bis[1-(3,4-dimethoxybenzyl)-3,4-dihydro-6,7-dimethoxy-1H-isoquinoline-2-propionate], dioxalate](https://cdn.globalso.com/jindunchem-med/28.png)
![6-tetra-O-acteyl-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydrofu-ran-3-yl]oxy]phenyl]](https://cdn.globalso.com/jindunchem-med/0ecf55f0-300x300.jpg)
