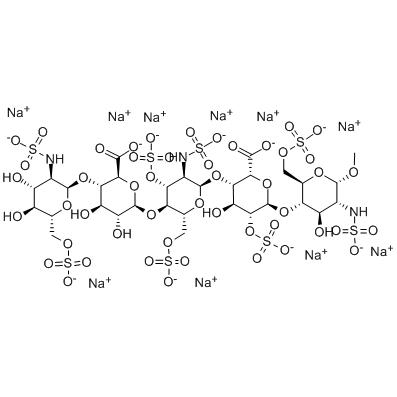
Quality Inspection for Industrial Reverse Osmosis - FondaparinSodiuM;Fondaparinux SodiuM Identification;Fondaparinux sodium N-4; – JIN DUN
Quality Inspection for Industrial Reverse Osmosis - FondaparinSodiuM;Fondaparinux SodiuM Identification;Fondaparinux sodium N-4; – JIN DUN
Quality Inspection for Industrial Reverse Osmosis - FondaparinSodiuM;Fondaparinux SodiuM Identification;Fondaparinux sodium N-4; – JIN DUN Detail:
Fondaparinux Biological Activity
| Describe | Fondaparinux sodium is an antithrombin-dependent factor Xa inhibitor。 |
| Related Categories | signal path >> Metabolic enzymes/proteases >> Factor Xa Research areas >> Cardiovascular diseases |
| Target | Factor Xa[1] |
| In vitro studies | Fondaparinux sodium is the first new anticoagulant that selectively targets factor Xa. For Fondaparinux, the IC50 value (anti-Xa IU/ml) of activated monocytes (ac-M) is 0.59±0.05, and monocyte-derived particles (MMP) is 0.17±0.03 [2]。 |
| In vivo research | Fondaparinux sodium has a linear, dose-dependent pharmacokinetic profile, which provides a highly predictable response. Fondaparinux sodium has 100% bioavailability, has a rapid onset of action, has a half-life of 14 to 16 hours, and can continue to resist thrombosis within 24 hours. The drug does not affect prothrombin time or activated partial thromboplastin time, nor does it affect platelet function or aggregation [1]。 |
| References | [1]. Bauer KA. et al. Fondaparinux sodium: a selective inhibitor of factor Xa. Am J Health Syst Pharm. 2001 Nov 1;58 Suppl 2:S14-7.[2]. Ben-Hadj-Khalifa S, et al. Differential coagulation inhibitory effect of fondaparinux, enoxaparin and unfractionated heparin in cell models of thrombin generation. Blood Coagul Fibrinolysis. 2011 Jul;22(5):369-73. |
Chemical and physical properties of Fondaparinux
| Molecular formula | C31H53N3Na10O49S8 |
| Molecular weight | 1738.16 |
| PSA | 900.82000 |
Fondaparinux is a new type of antithrombotic drug that has been approved by the FDA after heparin and low molecular weight heparin for the treatment and prevention of a variety of arteriovenous thrombosis.
Indications: Fondaparinux is used for patients undergoing major orthopedic surgery of lower limbs, such as hip fracture, major knee surgery or hip replacement, to prevent venous thromboembolism. It is used for the treatment of patients with unstable angina or non-ST-segment elevation myocardial infarction who undergo urgent (<120 minutes) invasive treatment (PCI) without indication. It is used for the treatment of patients with ST-segment elevation myocardial infarction who use thrombolysis or initially do not receive other forms of reperfusion therapy.
1)Glaxo’s continuous market promotion will place it in the core drug position in the subsequent clinical path of the Ministry of Health, the price reform of the National Development and Reform Commission, and the medical insurance of the Ministry of People’s Insurance.
A、The product has not yet entered the national medical insurance. In 2010, the medical insurance adjustment has just entered the local medical insurance of 16 provinces. Sales are in the initial stage and will gradually replace the low-molecular-weight heparin market; medical insurance provinces: Shaanxi, Shanxi, Inner Mongolia, Liaoning, Tibet, Yunnan, Guangdong , Guangxi, Hainan, Jiangsu, Gansu, Fujian, Jiangxi, Henan, Hubei, Beijing.
B、The indications are still increasing. GSK currently has 3 indications in the clinic. For example, the indications for stents are exclusively approved. Other heparins are not available. The clinical application of stents increases by 30% every year. With the approval of new indications, sales will increase. In theory, all diseases treated by heparin series products can be treated by this product.
C、Price advantage, the minimum price of this product in the US market is 132 US dollars each, in China is 168 yuan, the domestic price will not drop. If you do export, the international market has broad space;
2) The technical threshold is high, the raw material is synthesized in 75 steps, and the patent has expired for 5 years (article in 2014). It has not been approved by other manufacturers. The synthesis is extremely difficult. It will not be done in less than 10 years. The cycle is long, difficult, and investment. high. There are few domestic competitors, and foreign competitors have high raw material costs. Our goal is to continuously reduce raw material costs and replace Glaxo in raw material production.
Hengrui Pharmaceuticals Fondaparinux was approved as the first imitation in China 2018.
Guangdong Runxing Biotechnology Co., Ltd. has an annual output of 210 kg of fondaparinux sodium advanced intermediate N3, which will be filed in 2018.
Fondaparinux was originally developed by MYLAN IRELAND. Currently, Hengrui Pharmaceuticals, Borui Pharmaceuticals, Haisco.
Product detail pictures:
Related Product Guide:
go on to boost, to be certain item quality in line with market and buyer standard demands. Our firm has a excellent assurance procedure happen to be established for Quality Inspection for Industrial Reverse Osmosis - FondaparinSodiuM;Fondaparinux SodiuM Identification;Fondaparinux sodium N-4; – JIN DUN , The product will supply to all over the world, such as: New Orleans, Philippines, Berlin, Our team knows well the market demands in different countries, and is capable of supplying suitable quality products and solutions at the best prices to different markets. Our company has already set up a experienced, creative and responsible team to develop clients with the multi-win principle.
The company comply with the contract strict, a very reputable manufacturers, worthy a long-term cooperation.


![High definition Risperidone Generic - 2-butyl-5-nitro-3-benzofuranyl)[4-[3-(dibutylaMino)propoxy]phenyl] – JIN DUN](https://cdn.globalso.com/jindunchem-med/922e79ba.jpg)

![OEM Supply Ion Exchange Water Treatment - 4-[4-[(5S)-5-(Aminomethyl)-2-oxo-3-oxazolidinyl]phenyl]-3-morpholinone Hydrochloride – JIN DUN](https://cdn.globalso.com/jindunchem-med/dc3948321.jpg)