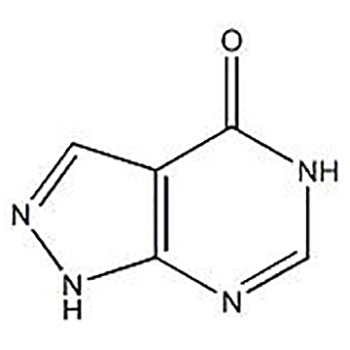
Popular Design for API Raw Material 99% Allopurinol CAS 315-30-0 Treatment of Gout
Popular Design for API Raw Material 99% Allopurinol CAS 315-30-0 Treatment of Gout
abide by the contract”, conforms into the market requirement, joins while in the market competition by its high quality as well as provides much more comprehensive and exceptional assistance for consumers to let them develop into significant winner. The pursue in the company, will be the clients’ satisfaction for Popular Design for API Raw Material 99% Allopurinol CAS 315-30-0 Treatment of Gout, Many years of work experience, we have realized the importance of providing good quality products and the best before-sales and after-sales services.
abide by the contract”, conforms into the market requirement, joins while in the market competition by its high quality as well as provides much more comprehensive and exceptional assistance for consumers to let them develop into significant winner. The pursue in the company, will be the clients’ satisfaction for China Allopurinol CAS 315-30-0 and Allopurinol CAS 315-30-0 Supplier, We’re seeking the chances to meet all the friends from both at home and abroad for the win-win cooperation. We sincerely hope to have long-term cooperation with all of you on the bases of mutual benefit and common development.
It is clinically used in primary and secondary Hyperuricemia, especially in patients with hyperuricemia, as well as in patients with renal insufficiency, and in patients with recurrent or chronic gout, as well as in patients with Hyperuricemia. Used in patients with Gouty nephropathy to relieve symptoms and reduce the formation of renal uric acid stones; gout stones; used in uric acid kidney stones and uric acid kidney disease.
This product and its metabolites can inhibit xanthine oxidase, so that hypoxanthine and xanthine cannot be converted into uric acid, that is, the synthesis of uric acid is reduced, thereby reducing the concentration of uric acid in the blood and reducing the concentration of urate in the bones, Joints and kidneys, drugs that can inhibit the synthesis of uric acid. This product can inhibit the activity of liver drug enzymes.
(1) . Primary and secondary hyperuricemia, especially those with excessive uric acid production, are also used for hyperuricemia with renal insufficiency;
(2) . For the treatment of gout, suitable for recurrent or chronic gout. For patients with gouty nephropathy, it can relieve symptoms and reduce the formation of uric acid stones in the kidneys;
(3) . Tophi;
(4) . Used for uric acid kidney stones and (or) uric acid nephropathy.
Standard:usp42
Assay:98-102%
Exterior:White or almost white powder
Package : 25kg/bag
There is bone marrow suppression, which can cause pancytopenia, and the drug should be discontinued if necessary. Drink plenty of water during the medication, and make the urine neutral or alkaline to excrete diuretic acid. This product must be applied after the acute inflammation of gouty arthritis disappears (usually about two weeks after the onset). During medication, blood picture and liver and kidney function should be checked regularly.
abide by the contract”, conforms into the market requirement, joins while in the market competition by its high quality as well as provides much more comprehensive and exceptional assistance for consumers to let them develop into significant winner. The pursue in the company, will be the clients’ satisfaction for Popular Design for API Raw Material 99% Allopurinol CAS 315-30-0 Treatment of Gout, Many years of work experience, we have realized the importance of providing good quality products and the best before-sales and after-sales services.
Popular Design for China Allopurinol CAS 315-30-0 and Allopurinol CAS 315-30-0 Supplier, We’re seeking the chances to meet all the friends from both at home and abroad for the win-win cooperation. We sincerely hope to have long-term cooperation with all of you on the bases of mutual benefit and common development.


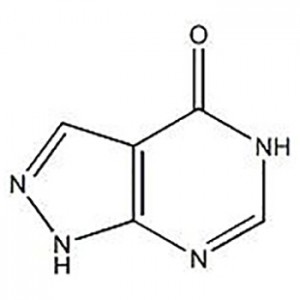
![Top Grade Copper Plating Chemical Dps Sodium 3-[[ (dimethylamino) Thioxomethyl]Thio]Propanesulphonate 18880-36-9](https://cdn.globalso.com/jindunchem-med/image271-300x300.png)