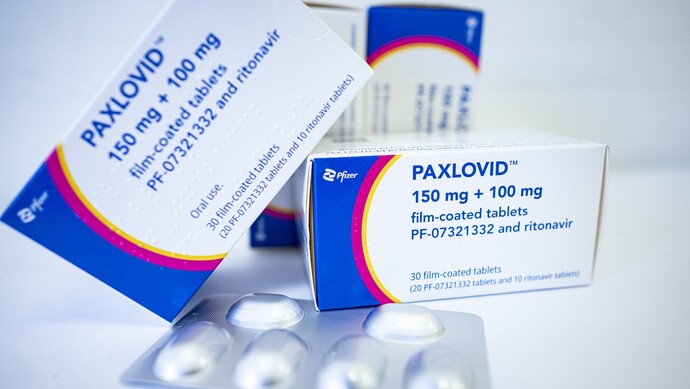
1.Thousands of dollars of COVID-19 Indian generic drugs sold out? look out!
Recently, a topic about “COVID-19 Indian generic drugs were sold for thousands of yuan in a box” appeared on the popular microblog search list. Some drugs were purchased on behalf of China News Network, and the stock had been sold out, so it was necessary to book them a week in advance. Doctors remind that drugs should not be purchased through informal channels. In addition, non high-risk groups do not need to use COVID-19 oral medicine.
The original price of a box of drugs is 2300 yuan, and the purchase price in India is 1600 yuan
“Now the orders are fully booked.” Several Indian drug purchasing agents told chinanews. com that their Pfizer COVID-19 oral Paxlovid generic drugs have been sold out recently. If necessary, they can only pay the deposit first, and the goods can be delivered in a week at the earliest, or next month at the slowest.
If the words “COVID-19 India”, “COVID-19 generic drugs” and so on are input on the e-commerce platform, consumers can quickly find the information of these purchasing agents, but they generally require to add WeChat friends, and then inform the specific goods information and purchase requirements.
The Paxlovid generic drugs sold by these agents include Primovir in green packaging and Paxista in blue packaging. The former is produced by Astrica, an Indian company, while the latter is produced by Azista, a subsidiary of the Indian pharmaceutical enterprise Hetero. At present, Primovir with green packaging has stopped production, and only Paxista with blue packaging is still on sale.
One agent introduced that the price of domestic spot mail was 1600 yuan per box, and that of overseas direct mail was 1200 yuan per box, 400 yuan cheaper. The purchase price of Paxlovid in China is 2300 yuan per box.
These generic drugs are hard to buy in stock at present. According to the above agent purchase, reservations are acceptable, and Indian direct mail will arrive in China in about 15-20 days. He also suggested that each person can only buy 2 boxes.
Doctors remind: It’s hard to tell the true from the false. Stay alert
In fact, Paxlovid has long been used in the treatment of COVID-19 infection in China, but it is mainly used in some designated hospitals and requires the authorization of doctors’ prescriptions. Not all hospitals have the drug.
It is worth noting that some generic drugs sold on a commission basis can be purchased without even providing a prescription. In this regard, professional doctors remind everyone to be vigilant.
“As professional doctors, we do not advocate buying foreign imitations, because it is difficult to tell the true from the false.” Zhang Jiming, deputy director of the National Infectious Diseases Medical Center and chief physician, professor and doctoral supervisor of Huashan Hospital affiliated to Fudan University, told chinanews. com that some of the patients he interviewed or their families bought imitations privately, and they were worried that they were buying fake drugs. He said that it was difficult to ensure the quality of generic drugs from the source and the preservation conditions of drugs when purchasing generic drugs from abroad.
Zhang Jiming introduced that COVID-19 oral medicine is mainly used for high-risk groups to prevent severe diseases, and it can be effective only when used early. Non high-risk groups do not need to rush to buy, and abuse may induce drug resistance. It is also unnecessary clinically, because most patients are asymptomatic or mild, and the course of disease is self limited.
He told chinanews. com that, as far as the situation in Shanghai is concerned, major hospitals, COVID-19 designated hospitals and other medical institutions generally have a certain amount of anti COVID-19 drugs, which are used by high risk groups who meet the indications and are infected with COVID-19, in order to prevent severe disease and reduce the mortality rate.
In addition, according to media reports, legal personnel remind that the Indian COVID-19 generic drugs sold online have not yet been approved in China. Under the current Drug Administration Law of the People’s Republic of China, although drugs that have been listed abroad but have not been approved in China are no longer simply identified as fake drugs, operators will still face administrative penalties for illegal imports of drugs.
2.Xinlitai has gone! Holding two 1 billion varieties, 11 types of Class 1 new drugs.
Recently, Xinlitai R&D pipeline has made new progress. Class 1 new drug SAL0133 tablets have been applied for clinical use. Preliminary statistical analysis results have been obtained from Phase Ib clinical trial of PCSK9 monoclonal antibody. Since 2022, Xinlitai has applied for IND/NDA for 7 innovative drugs, and innovation research and development has been steadily promoted. At present, Xinlitai has two 1 billion level varieties, 22 of which have been evaluated (8 are the first); 21 innovative drugs are under research, 6 of them are in the clinical stage of NDA or Phase III, and the innovation pipeline has entered the cashing period.
Broad spectrum COVID-19 Oral Medicine Appears! Xinlitai Enters COVID-19 Circuit
On December 20, Xinlitai announced that the clinical application of SAL0133, a small molecule innovative drug independently developed by the company, was accepted by the State Food and Drug Administration. SAL0133 is a powerful, broad-spectrum anti novel coronavirus 3CL protease (3CLpro) inhibitor independently innovated and developed by the company with independent intellectual property rights. At present, the clinical indication to be developed is to treat adult mild/common novel coronavirus pneumonia (COVID-19).
3CLpro plays an important role in RNA replication of novel coronavirus, mainly in the initial replication stage after the virus enters the host cell, inhibiting the activity of 3CLpro protease, which can effectively block virus replication and achieve the role of anti novel coronavirus.
SAL0133 has a clear mechanism of action, and has a strong, broad-spectrum anti COVID-19 effect. It is expected that it does not need to combine with the CYP3A4 inhibitor ritonavir, and the potential risk of drug interaction is low; It is expected to achieve clinical single drug use once a day and improve patient medication compliance. If it can be successfully developed and approved for marketing, it will provide patients with new drug choices to meet the unmet clinical needs.
The research and development of 3CL target anti COVID-19 oral small molecule drugs has attracted wide attention. At present, only Pfizer’s Paxlovid has been approved for marketing in the world. More than 10 domestic pharmaceutical enterprises and scientific research institutions have been conducting research and development of 3CL target anti COVID-19 drugs, including FB2001 of Frontier Biology, VV993 of Junshi Biology/Wangshan Wangshui, SIM0417 of Pioneer Pharmaceutical, RAY1216 tablets of Zhongsheng Pharmaceutical, GST-HG171 of Guangshengtang, etc.
With 2 major 1 billion varieties in hand, 7 innovative drugs this year welcome new progress
Towards the end of 2022, as a typical representative of R&D transformation in the pharmaceutical industry, Xinlitai successfully found its own innovative development path under pressure and adjustment.
In the first three quarters of 2022, the company’s revenue was 2.548 billion yuan, with a year-on-year growth of 16.5%, and its net profit was 539 million yuan, with a year-on-year growth of 37.64%. Good performance laid a solid foundation for subsequent development. The company expects that in 2022, Taijia (clopidogrel bisulfate tablets) will earn about 1 billion yuan, and Sinritan (alisartan tablets) will earn about 900 million yuan to 1 billion yuan. The company has 22 varieties, 8 of which are the first in China.
In 2019, the main variety of Xinlitai, Taijia, lost its bid in the 4+7 alliance centralized purchase, which forced Xinlitai to make changes. In the 2019 annual report, Xinlitai announced that “the research pipeline will be strategically optimized, and the anti-tumor biological analog and antibiotic projects in some clinical stages will be terminated”. In 2020, in order to further optimize the pipeline under research and focus on the R&D and promotion of innovative products, Xinlitai transferred the relevant rights and interests of dapoxetine hydrochloride, erlotinib hydrochloride, rivasaban and other projects, and received a transfer fee.
After some adjustments, at present, the generic drug pipeline of Xinritai only has the Class 4 marketing application for the imitation of Sakubatrovalsartan sodium tablets under review, and the supplementary application for consistency evaluation of cefuroxime sodium for injection and cefotaxime sodium for injection under review. It is worth mentioning that since July 2019, Xinlitai has not applied for new generic drugs for more than 3 years, and further concentrated on innovation and research.
Since 2022, Xinlitai has made continuous progress in the research and development of innovative drugs. On January 4, 2022, the listing application of CINRITAI’s HIF-PHI inhibitor Enasitar tablets was undertaken by CDE; Subsequently, the Company submitted clinical applications for five new drugs of Class 1, namely, recombinant human neuromodulin-1 anti HER3 antibody fusion protein injection, SAL0112 tablets, SAL008 injection, SAL0119 tablets and SAL0133 tablets; On November 3, the new class 2.3 improved drug alisartan and amlodipine tablets of SINRITAI submitted an application for listing, which is expected to form strategic synergy with the listed class 1.1 antihypertensive drug SINRITAI.
JinDun Medical has long-term scientific research cooperation and technology grafting with Chinese universities. With Jiangsu’s rich medical resources, it has long-term trade relations with India, Southeast Asia, South Korea, Japan and other markets. It also provides market and sales services in the whole process from intermediate to finished product API. Utilize the accumulated resources of Yangshi Chemical in fluorine chemistry to provide special chemical customization services for partners. Provide process innovation and impurity research services to target customers.
JinDun Medical insists on creating a team with dreams, making products with dignity, meticulous, rigorous, and go all out to be a trusted partner and friend of customers!One stop solution providers, customized R&D and customized production services for pharmaceutical intermediates and APIs, professional customized pharmaceutical production (CMO) and customized pharmaceutical R&D and production (CDMO) service providers. Jindun will accompany you to spend COVID-19.
Post time: Jan-28-2023