Lowest Price for Caudal Analgesia - 3′-Amino-2′-hydroxy-[1,1'-bipheny]-3-carboxylic acid – JIN DUN
Lowest Price for Caudal Analgesia - 3′-Amino-2′-hydroxy-[1,1'-bipheny]-3-carboxylic acid – JIN DUN
Lowest Price for Caudal Analgesia - 3′-Amino-2′-hydroxy-[1,1'-bipheny]-3-carboxylic acid – JIN DUN Detail:
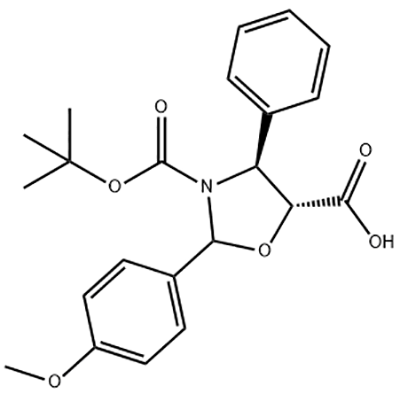
3′-Amino-2′-hydroxy-[1,1'-bipheny]-3-carboxylic acid is used as the intermediate of Eltrombopag .
Eltrombopag, developed by GlaxoSmithKline (GSK) in the UK and later jointly developed with Novartis in Switzerland, is the first and only approved small molecule non peptide TPO receptor agonist in the world. Eltrombopag was approved by the US FDA in 2008 for the treatment of idiopathic thrombocytopenic purpura (ITP), and in 2014 for the treatment of severe aplastic anemia (AA). It is also the first drug approved by the US FDA for the treatment of AA in recent 30 years.
In december2012, the US FDA approved Eltrombopag for the treatment of thrombocytopenia in patients with chronic hepatitis C (CHC), so that hepatitis C patients with poor prognosis due to low platelet count can start and maintain interferon based standard therapy for liver diseases. On february3,2014, GlaxoSmithKline announced that the FDA granted the breakthrough treatment drug qualification of Eltrombopag for the treatment of hemopenia in patients with severe chemicalbook aplastic anemia (SAA) who did not fully respond to immunotherapy. On August 24, 2015, the US FDA approved Eltrombopag for the treatment of thrombocytopenia in adults and children aged 1 year and over with chronic immune thrombocytopenia (ITP) who have insufficient response to corticosteroids, immunoglobulins or splenectomy. On january4,2018, Eltrombopag was approved to be listed in China for the treatment of primary immune thrombocytopenia (ITP).
Product detail pictures:
![Lowest Price for Caudal Analgesia - 3′-Amino-2′-hydroxy-[1,1'-bipheny]-3-carboxylic acid – JIN DUN detail pictures](https://cdn.globalso.com/jindunchem-med/image351.png)
Related Product Guide:
We emphasize progress and introduce new solutions into the market each year for Lowest Price for Caudal Analgesia - 3′-Amino-2′-hydroxy-[1,1'-bipheny]-3-carboxylic acid – JIN DUN , The product will supply to all over the world, such as: Porto, Australia, Somalia, Our organization. Situated inside the national civilized cities, the visitors is very easy, unique geographical and economic situations. We pursue a "people-oriented, meticulous manufacturing, brainstorm, construct brilliant" organization. hilosophy. Strict top quality management, fantastic service, reasonable cost in Myanmar is our stand on the premise of competition. If vital, welcome to make contact with us by our web page or telephone consultation, we've been likely to be pleased to serve you.
In our cooperated wholesalers, this company has the best quality and reasonable price, they are our first choice.


![Excellent quality Photoinitiator - Casp ungin Acetate;Caspofungin acetate;Cancidas;Caspofungin acetate [USAN:BAN:JAN]; – JIN DUN](https://cdn.globalso.com/jindunchem-med/fbe17385.jpg)