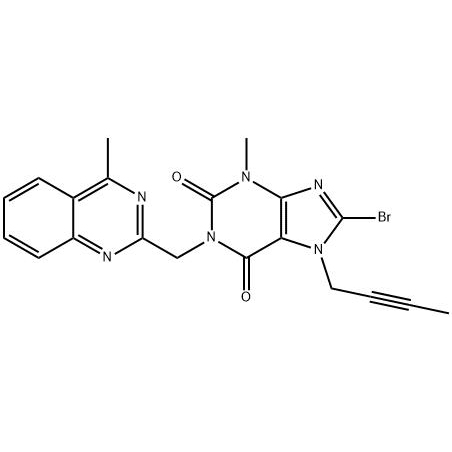
Low MOQ for Silicon Curable Resin - 8-Bromo-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3 – JIN DUN
Low MOQ for Silicon Curable Resin - 8-Bromo-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3 – JIN DUN
Low MOQ for Silicon Curable Resin - 8-Bromo-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3 – JIN DUN Detail:
Use: Intermediate for Linagliptin.
Use:Intermediate for Linagliptin
Executive standard: enterprise standard
Assay:98-102%
Exterior:White to light yellow powder
Package: 25kg/drum
To analyze the existing linagliptin and its key intermediate 8-bromo-7-(2-butynyl)-3,7-dihydro-3-methyl-1-[(4-methyl -2-quinazolinyl)methyl)-1H-purine-2,6-dione (11) synthesis method, find a synthetic route suitable for industrial production. Method: summarize the different synthetic routes. Results and conclusions: Route 2.2 has a relatively simple process and lower cost, which is more suitable for industrial production.
8-bromo-3,7-dihydro-3-methyl-1H-purine-2,6-dione is a key intermediate in the synthesis of the hypoglycemic drug linagliptin. The synthesis of 1 uses methyl urea and cyanoacetic acid as starting materials, and undergoes six-step reactions of condensation, cyclization, nitrosation, reduction, cyclization, and bromine with a total yield of 46.3%. The structures of all intermediates were confirmed by 1HNMR.
The present invention relates to a simple preparation method of high-purity linagliptin. Quinazoline, the key intermediate for the one-pot preparation of linagliptin 8 bromo 7 (2 butyne 1 base) 3,7 dihydro 3 methyl 1 [(4 methyl 2 quinazolinyl) methyl] 1H Purine 2,6 dione, the intermediate is separated by filtration, and then reacted with (R)3 aminopiperidine dihydrochloride to obtain a solution containing linagliptin. After the solution containing linagliptin is processed again, Deliraliptin pure product. The preparation of the key intermediate of the present invention adopts a one-pot method, which is convenient to operate and improves the yield. After the key intermediate is separated, it is reacted with (R)3 aminopiperidine dihydrochloride, thereby Obtaining high-purity linagliptin also meets the production and declaration requirements of pharmaceutical companies to the greatest extent.
Product detail pictures:
Related Product Guide:
We're committed to providing easy,time-saving and money-saving one-stop purchasing service of consumer for Low MOQ for Silicon Curable Resin - 8-Bromo-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3 – JIN DUN , The product will supply to all over the world, such as: Orlando, Swansea, South Korea, Our items have been obtained more and more recognition from foreign clients, and established long term and cooperative relationship with them. We`ll supply the best service for every customer and sincerely welcome friends to work with us and establish the mutual benefit together.
This supplier stick to the principle of "Quality first, Honesty as base", it is absolutely to be trust.


![High Quality Ultraviolet Water Treatment - 6-tetra-O-acteyl-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydrofu-ran-3-yl]oxy]phenyl] – JIN DUN](https://cdn.globalso.com/jindunchem-med/0ecf55f0.jpg)

![Wholesale Price Cefminox Sodium - 4-[4-[(5S)-5-(Aminomethyl)-2-oxo-3-oxazolidinyl]phenyl]-3-morpholinone Hydrochloride – JIN DUN](https://cdn.globalso.com/jindunchem-med/dc3948321.jpg)