Hot sale Wastewater Process - Entecavir USP Impurity B;Aids098045;Aids-098045;Etv;Enticavir;EntikaweiPian; – JIN DUN
Hot sale Wastewater Process - Entecavir USP Impurity B;Aids098045;Aids-098045;Etv;Enticavir;EntikaweiPian; – JIN DUN
Hot sale Wastewater Process - Entecavir USP Impurity B;Aids098045;Aids-098045;Etv;Enticavir;EntikaweiPian; – JIN DUN Detail:
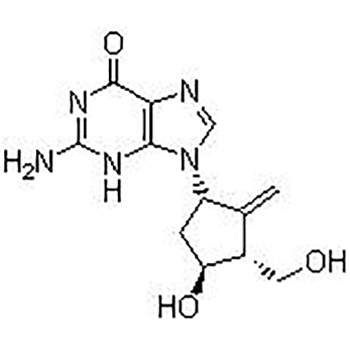
Entecavir(SQ 34676; BMS 200475)is selective and effective HBV Inhibitor. In HepG2 cells EC50 value is 3.75 nM. The latest anti-hepatitis B and anti-AIDS first-line drugs.
Entecavir effect
This product is a guanine nucleoside analog, which has an inhibitory effect on hepatitis B virus (HBV) polymerase. It can be phosphorylated into active triphosphate, and the half-life of triphosphate in the cell is 15 hours. By competing with HBV polymerase’s natural substrate deoxyguanosine triphosphate, entecavir triphosphate can inhibit all three activities of viral polymerase (reverse transcriptase): (1) HBV polymerase initiation; (2) pregenomic mRNA The formation of the negative strand of reverse transcription; (3) The synthesis of the positive strand of HBV DNA. The inhibitory constant (Ki) of entecavir triphosphate on HBV DNA polymerase is 0.0012μM. Entecavir triphosphate has weak inhibitory effect on cell α, β, δ DNA polymerase and mitochondrial γ DNA polymerase, with Ki value of 18 to 160 μM.
|
Describe |
Entecavir(SQ 34676; BMS 200475)is selective and effective HBV Inhibitor。In HepG2 cells EC50 value is 3.75 nM. |
|
Related categories |
signal path >> Anti-infective >> HBV Research areas >> Infect |
|
Target |
EC50: 3.75 nM (anti-HBV, HepG2 cell)[2] |
|
In vitro studies |
The EC50 of BMS-200475 to HBV is 3.75 nM. It is incorporated into HBV protein primers, and then the initiation step of reverse transcriptase is inhibited. The antiviral activity of BMS-200475 is significantly lower than other RNA and DNA viruses [1]. Compared with other deoxyguanosine analogs (penciclovir, ganciclovir, lobucavir and acyclovir) or lamivudine, entecavir is more likely to be phosphorylated to its active metabolite. The intracellular half-life of Entecavir is 15 hours [2]. |
|
In vivo research |
BMS-200475 is treated orally daily with a dose ranging from 0.02 to 0.5 mg/kg body weight for 1 to 3 months, which can effectively reduce the level of woodchuck hepatitis virus (WHV) viremia in chronically infected woodchucks [3 ]. |
|
Cell experiment |
BMS 200475 is prepared in phosphate buffered saline (PBS) and diluted with an appropriate medium containing 2% fetal bovine serum. HepG2 2.2.15 cells were seeded on a 12-well Biocoat collagen-coated plate at a density of 5×10 5 cells per well, and kept in a confluent state for 2 to 3 days, and then covered with 1 mL of BMS 200475 medium . HBV quantification was performed on the 10th day [1]. |
|
References |
[1]. Innaimo SF, et al. Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus. Antimicrob Agents Chemother. 1997 Jul;41(7):1444-9.[2]. Rivkin A, et al. A review of entecavir in the treatment of chronic hepatitis B infection. Curr Med Res Opin. 2005 Nov;21(11):1845-57.[3]. Genovesi EV, et al. Efficacy of the carbocyclic 2′-deoxyguanosine nucleoside BMS-200475 in the woodchuck model of hepatitis B virus infection. Antimicrob Agents Chemother. 1998 Dec;42(12):3209-18. |
Physical and chemical properties of Entecavir
|
Density |
1.8±0.1 g/cm3 |
|
Boiling point |
734.2ºC at 760 mmHg |
|
Melting point |
249-252ºC |
|
Molecular formula |
C12H15N5O3 |
|
Molecular weight |
277.279 |
|
Flash point |
397.9ºC |
|
Precise quality |
277.117493 |
|
PSA |
130.05000 |
|
LogP |
-0.96 |
|
Appearance traits |
White to off-white/yellow crystalline powder |
|
Refractive index |
1.837 |
|
Storage conditions |
-20°C Freezer |
Entecavir Customs
| Customs code | 2933990099 |
| Chinese Overview | 2933990099. Other heterocyclic compounds containing only nitrogen heteroatoms. Value-added tax rate: 17.0%. Tax rebate rate: 13.0%. Regulatory conditions: None. Most-favored nation tariff: 6.5%. General tariff: 20.0% |
| Declaration elements | Product name, ingredient content, use, hexamethylene chloride, please indicate the appearance, 6-caprolactam, please indicate the appearance, signing date |
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
Additional information:
Qu Qiang (PhD in clinical pharmacology, Department of Pharmacy, Xiangya Hospital, Central South University) and Wang Ruoguang (Doctor of Medicine, postdoctoral in biology, Changsha Ruoguang Medical Research Center) also wrote that: in the entecavir regimen, entecavir can be taken for several years or even 10 years. More than years. And it can be used by pregnant women, showing good safety.
Judging from these known molecular pharmacological mechanisms, Entecavir may have a broad-spectrum antiviral effect, not only effective against HBV.
We also often use Entecavir to treat viral respiratory infections in the clinic, and the effect is remarkable.
At present, in a retrospective analysis of more than 500 COVID19 patients in Hubei (a place), only one person took entecavir. The prescription time was in early January, and then the drug was stopped.
While supporting medical staff in Hubei, some grassroots doctors, or high-risk groups, currently using Entecavir to prevent COVID19, there is no case of infection. Suspected people taking entecavir, no one has progressed to be diagnosed with COVID19.
Patent issues:
There are mainly two processes, 06 process and 89 process. The expiration of the patent for the 06 process is too early (expires in 2023)! Although the cost of the process is low, the quality is relatively good, and the workers can be strong, personal advice: For chemical plants, use the 06 process not to be too arrogant; for pharmaceutical companies, don’t take a fluke and don’t be too risky .
In 2014, Chia Tai Tianqing achieved annual sales of 10 billion yuan by relying on generic drugs such as entecavir, of which entecavir accounted for 20%.
Since entecavir was approved for import in 2005, more than 100 domestic pharmaceutical companies have applied for the registration of the product; among them, the approved production companies include Jiangsu Zhengda Tianqing Pharmaceutical (first imitation), Hainan Zhonghe Pharmaceutical, and Fujian Guangxi Pharmaceutical Co., Ltd. Shengtang Pharmaceutical, Jiangxi Qingfeng Pharmaceutical, Suzhou Dawnrays Pharmaceutical, Shanghai Qingsong Pharmaceutical, Sichuan Haisco Pharmaceutical, Shandong Lukang, Zhejiang Huasheng Biopharmaceutical, Changzhi Sanbao Biochemical.
Pharmaceutical, Anhui Baker Biopharmaceutical, Hunan Concord Pharmaceutical Industry, Beijing Xiehe Pharmaceutical Factory, Beijing Baiao Pharmaceutical, Xinlitai Pharmaceutical, Dongyangguang, etc.; further from the date of contract, this product has entered the ranks of popular declared varieties since 2007.
Product detail pictures:
Related Product Guide:
We usually perform being a tangible workforce making sure that we will give you the most beneficial excellent plus the finest selling price for Hot sale Wastewater Process - Entecavir USP Impurity B;Aids098045;Aids-098045;Etv;Enticavir;EntikaweiPian; – JIN DUN , The product will supply to all over the world, such as: Egypt, Bandung, Serbia, The company has numbers of foreign trade platforms, which are Alibaba,Globalsources,Global Market,Made-in-china. "XinGuangYang" HID brand products sell very well in Europe, America, Middle East and other regions more than 30 countries.
In general, we are satisfied with all aspects, cheap, high-quality, fast delivery and good procuct style, we will have follow-up cooperation!



![Fast delivery Iron Water Filter System - 2-butyl-5-nitro-3-benzofuranyl)[4-[3-(dibutylaMino)propoxy]phenyl] – JIN DUN](https://cdn.globalso.com/jindunchem-med/922e79ba.jpg)