Factory wholesale Stopping Risperidone Cold Turkey - 2-butyl-5-nitro-3-benzofuranyl)[4-[3-(dibutylaMino)propoxy]phenyl] – JIN DUN
Factory wholesale Stopping Risperidone Cold Turkey - 2-butyl-5-nitro-3-benzofuranyl)[4-[3-(dibutylaMino)propoxy]phenyl] – JIN DUN
Factory wholesale Stopping Risperidone Cold Turkey - 2-butyl-5-nitro-3-benzofuranyl)[4-[3-(dibutylaMino)propoxy]phenyl] – JIN DUN Detail:
It is clinically used in primary and secondary Hyperuricemia, especially in patients with hyperuricemia, as well as in patients with renal insufficiency, and in patients with recurrent or chronic gout, as well as in patients with Hyperuricemia. Used in patients with Gouty nephropathy to relieve symptoms and reduce the formation of renal uric acid stones; gout stones; used in uric acid kidney stones and uric acid kidney disease.
The new anti-arrhythmic drug Atrial fibrillation (AF) is a potentially life-threatening disease. Due to the aging of the population, the global incidence and mortality of atrial fibrillation are increasing, and it has become a new public Health problems. There are 2.5 million patients with atrial fibrillation in the United States and 4.5 million in the European Union. According to statistics, the prevalence of atrial fibrillation among people over 30 years old in my country is 0.77%, which increases with age, and males are higher than females (0.9%:0.7%). The diseases that cause atrial fibrillation mainly include hypertension, coronary heart disease, valvular heart disease, spontaneous dilated cardiomyopathy, and heart failure. The main symptom of patients with atrial fibrillation is irregular ventricular rate, and heart rate control occupies a certain position in the acute treatment and maintenance of symptoms. Amiodarone plays a good role in controlling heart rate and restoring sinus rhythm, but the adverse effects of target organs related to the iodine component in its structure limit its clinical application, such as damage to the lung, thyroid, and liver. The above-mentioned adverse reactions and its structure The iodine in is closely related. The new antiarrhythmic drug Dronedarone (Dronedarone) was obtained by modifying the structure of amiodarone. Dronedarone has electrophysiological properties similar to amiodarone, but does not contain iodine in its structure, so it can avoid serious extra-cardiac side effects caused by iodine Chemicalbook. Dronedarone was approved for marketing by the U.S. Food and Drug Administration (FDA) in July 2009, and was approved for marketing in the European Union in 2009. It is also the first new antiarrhythmic drug approved in the European Union in 10 years. Dronedarone is N-[2-butyl-3-[4-[3-(dibutylamino)propoxy]phenyl]-5-benzofuranyl]-methanesulfonamide, which is to reduce A benzofuran derivative obtained by structural modification of amiodarone, which is not a cardiac adverse reaction. It removes the iodine atom in amiodarone to reduce the toxicity of the thyroid and other organs; adds a methylsulfonyl group on the benzofuran side to reduce lipophilicity, thereby shortening the half-life of the drug and reducing the tissue accumulation effect and possibility of the drug Of neurotoxicity. Both the 2011 US version and the 2010 European version of the Atrial Fibrillation Guidelines recommend dronedarone for the treatment of atrial fibrillation. Dronedarone is safe and effective in treating ventricular rate and maintaining sinus rate in various patients without severe AF. It can be an effective drug to replace amiodarone. Dronedarone can also reduce the death of AF patients and even persistent AF patients. Therefore, it is expected to become the first-line medication for AF patients without severe heart failure. Moreover, the use of anticoagulants in middle-high-risk AF patients is beneficial to the use of dronedarone.
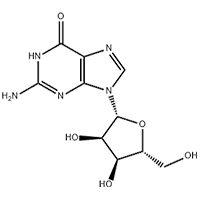
Use:Intermediate for Dronedarone hydrochloride
Standard:enterprise standard
Assay:≥98%
Exterior: Yellow oily liquid
Package: 25kg/drum
Product detail pictures:
![Factory wholesale Stopping Risperidone Cold Turkey - 2-butyl-5-nitro-3-benzofuranyl)[4-[3-(dibutylaMino)propoxy]phenyl] – JIN DUN detail pictures](https://cdn.globalso.com/jindunchem-med/922e79ba.jpg)
Related Product Guide:
Quality First,and Customer Supreme is our guideline to provide the best service to our customers.Nowadays, we are trying our best to become one of the best exporters in our field to meet customers more need for Factory wholesale Stopping Risperidone Cold Turkey - 2-butyl-5-nitro-3-benzofuranyl)[4-[3-(dibutylaMino)propoxy]phenyl] – JIN DUN , The product will supply to all over the world, such as: Croatia, Greece, Juventus, Our company promises: reasonable prices, short production time and satisfactory after-sales service, we also welcome you to visit our factory at any time you want. Wish we have a pleasant and long terms business together!!!
This is the first business after our company establish, products and services are very satisfying, we have a good start, we hope to cooperate continuous in the future!


![Leading Manufacturer for Non Chemical Water Treatment For Cooling Towers - 6-tetra-O-acteyl-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydrofu-ran-3-yl]oxy]phenyl] – JIN DUN](https://cdn.globalso.com/jindunchem-med/0ecf55f0.jpg)