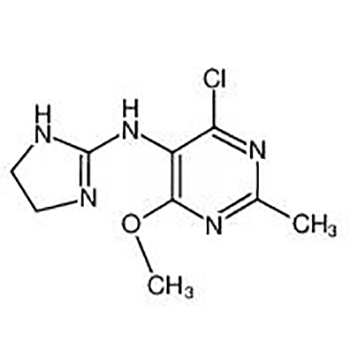
Factory Free sample High Pressure Hydrogenolysis Reaction - 4-chloro-n-(4,5-dihydro-1h-imidazol-2-yl)-6-methoxy-2-methyl-5-pyrimidinamin; – JIN DUN
Factory Free sample High Pressure Hydrogenolysis Reaction - 4-chloro-n-(4,5-dihydro-1h-imidazol-2-yl)-6-methoxy-2-methyl-5-pyrimidinamin; – JIN DUN
Factory Free sample High Pressure Hydrogenolysis Reaction - 4-chloro-n-(4,5-dihydro-1h-imidazol-2-yl)-6-methoxy-2-methyl-5-pyrimidinamin; – JIN DUN Detail:
It works by stimulating central presynaptic alpha 2- receptors. Its hypotensive effect is similar to that of nifedipine, a calcium antagonist, and captopril, an ACE inhibitor. For essential hypertension.
This product should adopt the principle of individualized medication. Generally start from the lowest dose, that is, 0.2mg, once a day, taken in the morning. Dosage adjustments should be made at three-week intervals until satisfactory results are obtained. The usual dose is 0.2mg once, twice a day (morning and evening). The maximum dose per time shall not exceed 0.4mg, and the maximum daily dose shall not exceed 0.6mg. For patients with mild or moderate renal insufficiency, the single dose should not exceed 0.2 mg or the daily dose should not exceed 0.4 mg.
This product is an α2-receptor agonist, which is an imidazoline antihypertensive drug. Used for moderate and severe primary and secondary hypertension.
Because it inhibits the secretion and movement of the gastrointestinal tract, it is also suitable for hypertensive patients suffering from ulcer disease. The combination of this medicine and diuretics can significantly improve the efficacy. It is a powerful central antihypertensive drug and a second-line antihypertensive drug. It is mainly administered locally in ophthalmology. This product has the effect of reducing intraocular pressure, and does not affect the pupil and eye adjustment mechanism. It can constrict blood vessels in the eye, reduce the production of aqueous humor, and achieve the effect of reducing intraocular pressure. It starts to take effect 15 minutes after the eye drops and can maintain the effect for about 8 hours.
Standard:EP10
Assay content:97.5-102.0%
Exterior: White or almost while powder
Package: 1kg/bag or as required by customers
Product detail pictures:
Related Product Guide:
We can easily usually fulfill our respected customers with our very good top quality, very good price tag and excellent support due to we have been more expert and much more hard-working and do it in cost-effective way for Factory Free sample High Pressure Hydrogenolysis Reaction - 4-chloro-n-(4,5-dihydro-1h-imidazol-2-yl)-6-methoxy-2-methyl-5-pyrimidinamin; – JIN DUN , The product will supply to all over the world, such as: Singapore, Australia, Grenada, We warmly welcome your patronage and will serve our clients both at home and abroad with products of superior quality and excellent service geared to the trend of further development as always. We believe you will benefit from our professionalism soon.
Reasonable price, good attitude of consultation, finally we achieve a win-win situation,a happy cooperation!


![Factory supplied Sodium Carbonate Water Treatment - 6-tetra-O-acteyl-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydrofu-ran-3-yl]oxy]phenyl] – JIN DUN](https://cdn.globalso.com/jindunchem-med/0ecf55f0.jpg)
![professional factory for Hepatitis Ab And C - Casp ungin Acetate;Caspofungin acetate;Cancidas;Caspofungin acetate [USAN:BAN:JAN]; – JIN DUN](https://cdn.globalso.com/jindunchem-med/fbe17385.jpg)
![China OEM Ozone Water Disinfection - 5-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]- 5,6,7,7a-tetrahydrothieno[3,2-c]pyridin-2 (4h)-one – JIN DUN](https://cdn.globalso.com/jindunchem-med/image41.png)