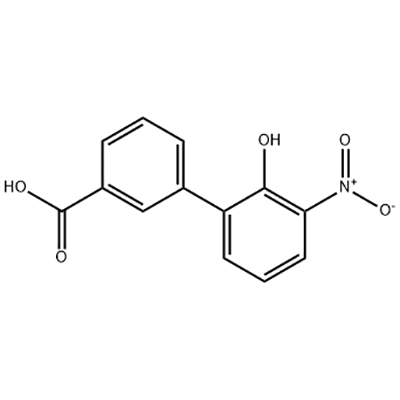
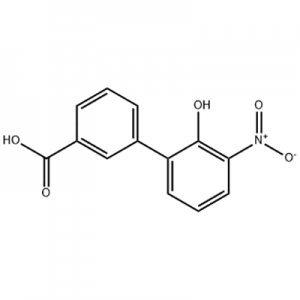
Europe style for 3′-Hydroxy-3-Biphenylcarboxylic Acid Citric Acid 77-92-9
Europe style for 3′-Hydroxy-3-Biphenylcarboxylic Acid Citric Acid 77-92-9
Quality First,and Client Supreme is our guideline to deliver the very best assistance to our shoppers.These days, we have been trying our greatest to be amongst the ideal exporters inside our field to fulfill consumers extra will need for Europe style for 3′-Hydroxy-3-Biphenylcarboxylic Acid Citric Acid 77-92-9, Through additional than 8 years of small business, we have now accumulated rich experience and advanced technologies during the manufacturing of our products.
Quality First,and Client Supreme is our guideline to deliver the very best assistance to our shoppers.These days, we have been trying our greatest to be amongst the ideal exporters inside our field to fulfill consumers extra will need for China 77-92-9 and CAS 77-92-9, Based on our automatic production line, steady material purchase channel and quick subcontract systems have been built in mainland China to meet customer’s wider and higher requirement in recent years. We have been looking forward to cooperating with more clients worldwide for common development and mutual benefit!Your trust and approval are the best reward for our efforts. Keeping honest, innovative and efficient, we sincerely expect that we can be business partners to create our brilliant future!
2′-hydroxy-3′-nitro-3-biphenylcarboxylic acid is used as the intermediate of Eltrombopag .
Eltrombopag, developed by GlaxoSmithKline (GSK) in the UK and later jointly developed with Novartis in Switzerland, is the first and only approved small molecule non peptide TPO receptor agonist in the world. Eltrombopag was approved by the US FDA in 2008 for the treatment of idiopathic thrombocytopenic purpura (ITP), and in 2014 for the treatment of severe aplastic anemia (AA). It is also the first drug approved by the US FDA for the treatment of AA in recent 30 years.
In december2012, the US FDA approved Eltrombopag for the treatment of thrombocytopenia in patients with chronic hepatitis C (CHC), so that hepatitis C patients with poor prognosis due to low platelet count can start and maintain interferon based standard therapy for liver diseases. On february3,2014, GlaxoSmithKline announced that the FDA granted the breakthrough treatment drug qualification of Eltrombopag for the treatment of hemopenia in patients with severe chemicalbook aplastic anemia (SAA) who did not fully respond to immunotherapy. On August 24, 2015, the US FDA approved Eltrombopag for the treatment of thrombocytopenia in adults and children aged 1 year and over with chronic immune thrombocytopenia (ITP) who have insufficient response to corticosteroids, immunoglobulins or splenectomy. On january4,2018, Eltrombopag was approved to be listed in China for the treatment of primary immune thrombocytopenia (ITP).
Quality First,and Client Supreme is our guideline to deliver the very best assistance to our shoppers.These days, we have been trying our greatest to be amongst the ideal exporters inside our field to fulfill consumers extra will need for Europe style for 3′-Hydroxy-3-Biphenylcarboxylic Acid Citric Acid 77-92-9, Through additional than 8 years of small business, we have now accumulated rich experience and advanced technologies during the manufacturing of our products.
Europe style for China 77-92-9 and CAS 77-92-9, Based on our automatic production line, steady material purchase channel and quick subcontract systems have been built in mainland China to meet customer’s wider and higher requirement in recent years. We have been looking forward to cooperating with more clients worldwide for common development and mutual benefit!Your trust and approval are the best reward for our efforts. Keeping honest, innovative and efficient, we sincerely expect that we can be business partners to create our brilliant future!