Casp ungin Acetate;Caspofungin acetate;Cancidas;Caspofungin acetate [USAN:BAN:JAN];
Casp ungin Acetate;Caspofungin acetate;Cancidas;Caspofungin acetate [USAN:BAN:JAN];
Caspofungin Acetate Biological Activity
| Describe | Caspofungin Acetate is an antifungal drug that can non-competitively inhibit the synthesis of 1,3-β-d glucan synthase。 |
| Related categories | signal path >> Anti-infective >> Fungus
Research areas >> Infect |
| In vivo research | Mice injected with caspofungin had no significant changes in their ERG waveform at a vitreous concentration of 0.41 to 4.1 μM, and their retina had no detectable morphological changes or cell loss. At a vitreous concentration of 41 μM, caspofungin reduced the amplitude of the a wave, b wave and scotopic threshold response of ERG, and also caused a decrease in the number of cells in the ganglion cell layer [1]. Carprofungin (8 mg/kg) or amphotericin B 1 mg/kg, intraperitoneal injection once a day 30 h after infection for 7 consecutive days, relative to vehicle control treatment, 100% survival on day 28, resulting in daytime 100% mortality rate 11, after infectious challenge. Compared with the vehicle control treatment on day 5, caspofungin reduced the recovery of viable Candida in kidney and brain tissue when the control load reached its peak. Mice treated with caspofungin at a dose of 2 mg/kg or higher had significantly lower brain load on day 5 than mice treated with amphotericin B. Amphotericin B and caspofungin treatment reduced renal fungal burden by 1.7 log CFU/g and 2.46 to 3.64 log CFU/g, respectively [2]。 |
| Animal experiment | Antifungal therapy was started 30 hours after the infectious challenge and was given by intraperitoneal (ip) injection once a day for 7 days. Mice were treated with 1, 2, 4, or 8 mg/kg/day caspofungin, 1 mg/kg/day amphotericin B or vehicle control (sterile distilled water). The efficacy of the model was evaluated in three ways: by monitoring the survival rate of 10 animals in each treatment group, by monitoring the Candida load in the kidney and brain tissues of the second group of treated animals, and by histologically evaluating the kidneys. And brain. The third group of treated animals. The mice were euthanized by CO 2 inhalation and cultured at 30 hours (vehicle-treated control only) and on day 5 (24 hours after the 4th dose), 8 (24 hours after the last dose), and 14, 21 hours And histological tissue sampling. (Only caspofungin treatment), and 28 after the attack. |
| References | [1]. Mojumder DK, et al. Evaluating retinal toxicity of intravitreal caspofungin in the mouse eye. Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5796-803.
[2]. Flattery, Amy M. et al. Efficacy of caspofungin in a juvenile mouse model of central nervous system candidiasis. Antimicrobial Agents and Chemotherapy (2011), 55(7), 3491-3497. |
Chemical and physical properties of caspofungin acetate
| Boiling point | 1408.1ºC at 760 mmHg |
| Molecular formula | C56H96N10O19 |
| Molecular weight | 1093.31000 |
| Precise quality | 1092.64000 |
| PSA | 412.03000 |
| LogP | 0.06150 |
| Appearance traits | white to beige |
| Vapor pressure | 0mmHg at 25°C |
| Storage conditions | 20°C |
| Water solubility | H2O: soluble15mg/mL (clear solution) |
Caspofungin Acetate Customs
| Customs code | 2933990099 |
| Chinese Overview | 2933990099. Other heterocyclic compounds containing only nitrogen heteroatoms. Value-added tax rate: 17.0%. Tax rebate rate: 13.0%. Regulatory conditions: None. Most-favored nation tariff: 6.5%. General tariff: 20.0% |
| Declaration elements | Product name, ingredient content, use, hexamethylene chloride, please indicate the appearance, 6-caprolactam, please indicate the appearance, signing date |
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
Production Process
Caspofungin needs to be prepared by fermentation semi-synthetic technology. The crude product of the main ring needs to be obtained through fermentation technology, and then the intermediate product is obtained through separation and purification, and then the intermediate product is used as the starting material to complete the side chain splicing using synthetic technology to finally obtain the target Element. Because the "fermented semi-synthetic" products need to go through multiple technical links such as fermentation, separation and purification, synthesis, etc., the technical route and process parameter control are very complicated in Chemicalbook. In addition, the regulatory requirements for the registration of caspofungin, a fermented semi-synthetic product, are also extremely high. The application documents need to start from the fermentation source, not only to systematically study strain cultivation, fermentation technology, and purification technology, but also to explore the synthesis process. Route, conditions, and control of impurities in the process, because the final product is quite fragile, any carelessness will wipe out all previous efforts, and the technical difficulty and cost are not insignificant.
It is precisely because of high technical barriers and a good competitive landscape that Hengrui Caspofungin has maintained a relatively good price space after its listing.
Patent protection expired in 2014
Sales of terminal caspofungin injections in public medical institutions in China (unit: ten thousand yuan)
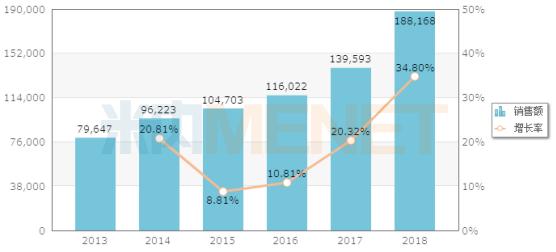
Up to now, only the generic drugs of Hengrui Pharmaceuticals, Chia Tai Tianqing, Borui Pharmaceuticals, and Haisco have been approved for listing. Huadong Medicine?
The original research company of caspofungin acetate is Merck, and the listing applications of 6 companies are under review and approval, namely Qilu Pharmaceutical, Nanjing Yinxing Pharmaceutical, Sihuan Pharmaceutical, Osaikang Pharmaceutical, Sino-US Huadong Pharmaceutical, and Tian Wei Biopharmaceuticals.

JIN DUN Medical has ISO qualification and meets GMP production standards, employed domestic and foreign drug synthesis experts with rich experience to guide company's R&D.




TECHNOL OGY ADVANTAGES
●High Pressure Catalytic Hydrogenation . High Pressure Hydrogenolysis Reaction . Cryogenic Reaction (<-78%C)
●Aromatic Heterocyclic Synthesis
●Rearrangement Reaction
●Chiral Resolution
●Heck, Suzuki,Negishi,Sonogashira . Gignard Reaction
Equipments
Our Lab has various experimental and testing equipments, such as: NMR (Bruker 400M)、HPLC、chiral-HPLC、LC-MS、LC-MS/MS (API 4000)、IR、UV、GC、GC-MS, Chromatography, Microwave Synthesizer, Parallel Synthesizer, Differential Scanning Calorimeter (DSC), Electron Microscope...
R&D Team
Jindun Medical has a group of professional R&D personnel, and employs many domestic and foreign drug synthesis experts to guide R&D, making our synthesis more accurate and efficient.

We have helped several top domestic pharmaceutical companies, such as Hansoh, Hengrui and HEC Pharm. Here we will show part of them.
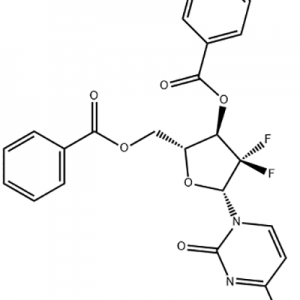
Customzation Case One:
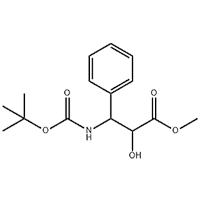
Cas No.: 110351-94-5
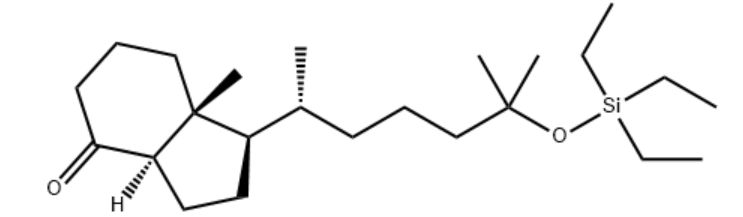
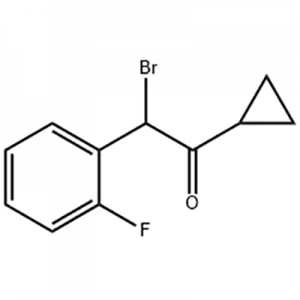
Customzation Case Two:
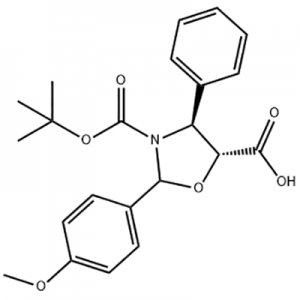
Cas No.: 144848-24-8
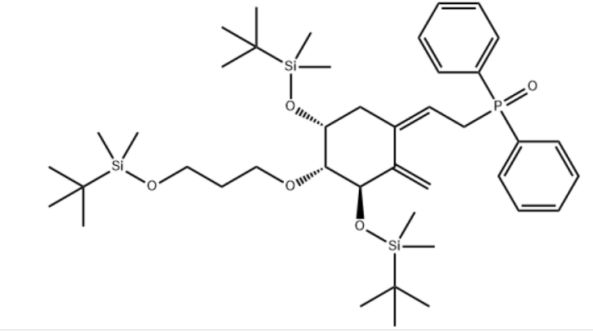
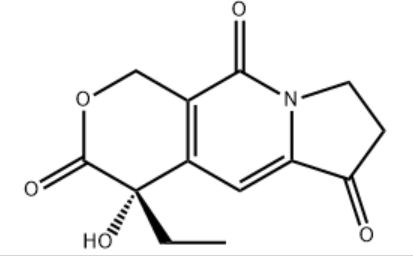
Customzation Case Three:
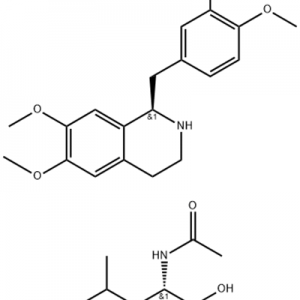
Cas No.: 200636-54-0
1.Customize New Intermediates or APIs. Same as above case sharing, customers have the demands for specific Intermediates or APIs, and they can not find the needed products in the market, then we can help to Customize.
2.Process Optimization for Old Products. Our team will help to Optimize and improve such production whose reaction route is old, production cost is high, and the efficiency is low. We can provide full documentation for technology transfer and process improvement, help customer for a more efficient production.
From drug targets to INDs, JIN DUN Medical provides you with one-stop personalized R&D solutions.
JIN DUN Medical insists on creating a team with dreams, making dignified products, meticulous, rigorous, and going all out to be a trusted partner and friend of customers!


![Casp ungin Acetate;Caspofungin acetate;Cancidas;Caspofungin acetate [USAN:BAN:JAN]; Featured Image](https://cdn.globalso.com/jindunchem-med/fbe17385.jpg)
![Casp ungin Acetate;Caspofungin acetate;Cancidas;Caspofungin acetate [USAN:BAN:JAN];](https://cdn.globalso.com/jindunchem-med/fbe17385-300x300.jpg)