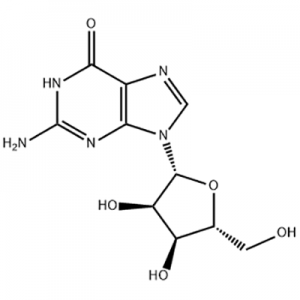
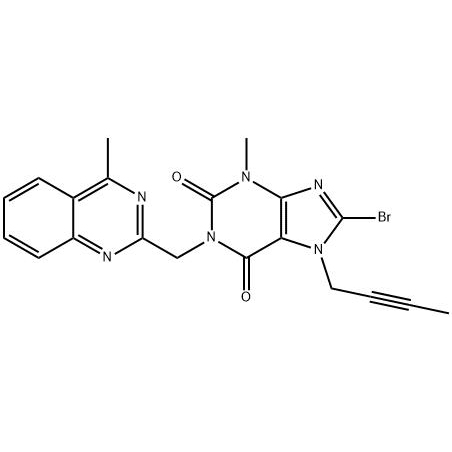
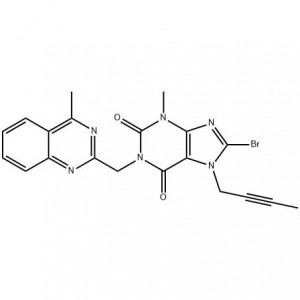
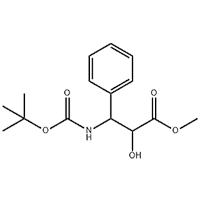
8-Bromo-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3
8-Bromo-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3
Use: Intermediate for Linagliptin.
Use:Intermediate for Linagliptin
Executive standard: enterprise standard
Assay:98-102%
Exterior:White to light yellow powder
Package: 25kg/drum
To analyze the existing linagliptin and its key intermediate 8-bromo-7-(2-butynyl)-3,7-dihydro-3-methyl-1-[(4-methyl -2-quinazolinyl)methyl)-1H-purine-2,6-dione (11) synthesis method, find a synthetic route suitable for industrial production. Method: summarize the different synthetic routes. Results and conclusions: Route 2.2 has a relatively simple process and lower cost, which is more suitable for industrial production.
8-bromo-3,7-dihydro-3-methyl-1H-purine-2,6-dione is a key intermediate in the synthesis of the hypoglycemic drug linagliptin. The synthesis of 1 uses methyl urea and cyanoacetic acid as starting materials, and undergoes six-step reactions of condensation, cyclization, nitrosation, reduction, cyclization, and bromine with a total yield of 46.3%. The structures of all intermediates were confirmed by 1HNMR.
The present invention relates to a simple preparation method of high-purity linagliptin. Quinazoline, the key intermediate for the one-pot preparation of linagliptin 8 bromo 7 (2 butyne 1 base) 3,7 dihydro 3 methyl 1 [(4 methyl 2 quinazolinyl) methyl] 1H Purine 2,6 dione, the intermediate is separated by filtration, and then reacted with (R)3 aminopiperidine dihydrochloride to obtain a solution containing linagliptin. After the solution containing linagliptin is processed again, Deliraliptin pure product. The preparation of the key intermediate of the present invention adopts a one-pot method, which is convenient to operate and improves the yield. After the key intermediate is separated, it is reacted with (R)3 aminopiperidine dihydrochloride, thereby Obtaining high-purity linagliptin also meets the production and declaration requirements of pharmaceutical companies to the greatest extent.

JIN DUN Medical has ISO qualification and meets GMP production standards, employed domestic and foreign drug synthesis experts with rich experience to guide company's R&D.




TECHNOL OGY ADVANTAGES
●High Pressure Catalytic Hydrogenation . High Pressure Hydrogenolysis Reaction . Cryogenic Reaction (<-78%C)
●Aromatic Heterocyclic Synthesis
●Rearrangement Reaction
●Chiral Resolution
●Heck, Suzuki,Negishi,Sonogashira . Gignard Reaction
Equipments
Our Lab has various experimental and testing equipments, such as: NMR (Bruker 400M)、HPLC、chiral-HPLC、LC-MS、LC-MS/MS (API 4000)、IR、UV、GC、GC-MS, Chromatography, Microwave Synthesizer, Parallel Synthesizer, Differential Scanning Calorimeter (DSC), Electron Microscope...
R&D Team
Jindun Medical has a group of professional R&D personnel, and employs many domestic and foreign drug synthesis experts to guide R&D, making our synthesis more accurate and efficient.

We have helped several top domestic pharmaceutical companies, such as Hansoh, Hengrui and HEC Pharm. Here we will show part of them.
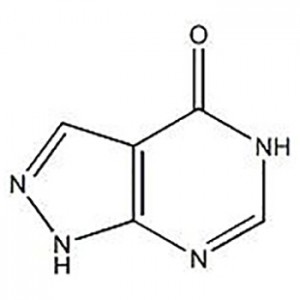
Customzation Case One:
Cas No.: 110351-94-5
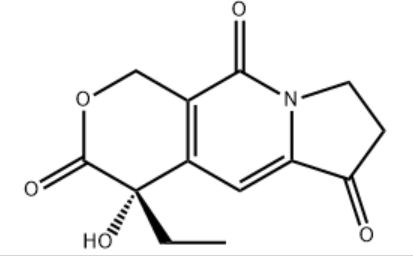
Customzation Case Two:
Cas No.: 144848-24-8
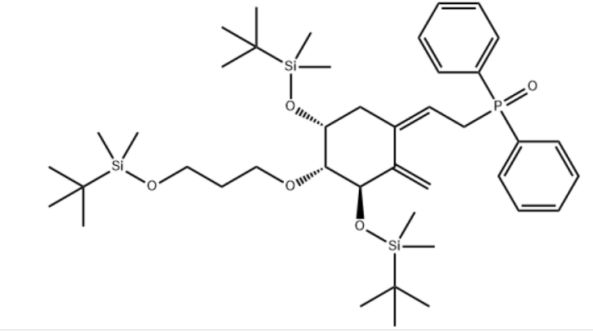
Customzation Case Three:
Cas No.: 200636-54-0
1.Customize New Intermediates or APIs. Same as above case sharing, customers have the demands for specific Intermediates or APIs, and they can not find the needed products in the market, then we can help to Customize.
2.Process Optimization for Old Products. Our team will help to Optimize and improve such production whose reaction route is old, production cost is high, and the efficiency is low. We can provide full documentation for technology transfer and process improvement, help customer for a more efficient production.
From drug targets to INDs, JIN DUN Medical provides you with one-stop personalized R&D solutions.
JIN DUN Medical insists on creating a team with dreams, making dignified products, meticulous, rigorous, and going all out to be a trusted partner and friend of customers!


![4-[4-[(5S)-5-(Aminomethyl)-2-oxo-3-oxazolidinyl]phenyl]-3-morpholinone Hydrochloride](https://cdn.globalso.com/jindunchem-med/dc3948321-300x300.jpg)
![2-butyl-5-nitro-3-benzofuranyl)[4-[3-(dibutylaMino)propoxy]phenyl]](https://cdn.globalso.com/jindunchem-med/922e79ba.jpg)